Mutations vs. Science - How Vorasidenib Battles Mutations
Published on March 12, 2024
Gliomas, a class of tumors originating in the central nervous system, pose a significant challenge in cancer fields. These insidious growths from the very glial cells that support and protect neurons, exhibit a diverse range of characteristics and complexities. They can manifest in various regions of the brain and spinal cord, making their diagnosis and treatment a nuanced endeavor. One defining aspect of gliomas is their association with numerous mutations, contributing to the individualized nature of glioma development, and demonstrating the need for personalized and targeted therapeutic approaches.
One such mutation is the isocitrate dehydrogenase (IDH) mutation. Identified in over 80% of glioma cases, the mutated form of the IDH enzyme contributes to altered cellular metabolism, fostering an environment conducive to tumor growth. This genetic aberration not only serves as a diagnostic marker but also plays a crucial role in shaping the aggressive nature of gliomas (causing weakness, seizures, and rarely even death).
To battle the negative effects of these gliomas, a targeted drug therapy known as Vorasidenib was developed for blocking the activity of the abnormal proteins (IDH1 and IDH2) developed due to the IDH mutation. Here’s a figure that details the entire process - from mutation to drug action:
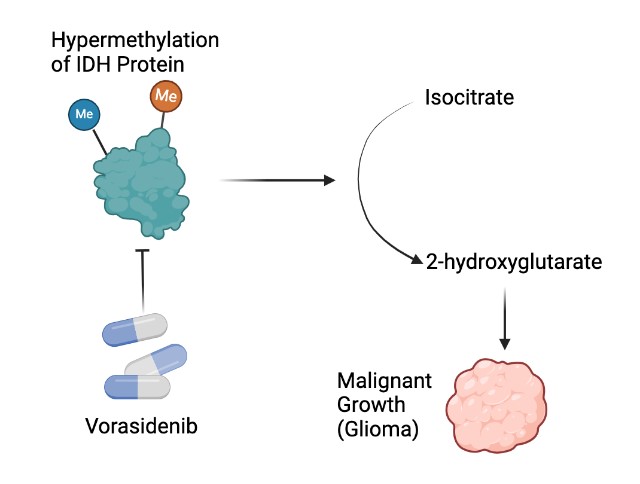
In normal cellular metabolism, IDH enzymes catalyze the conversion of isocitrate to alpha-ketoglutarate. However, mutations in the IDH genes, particularly IDH1 and IDH2, lead to a gain-of-function alteration, transforming the enzymatic activity. Instead of producing alpha-ketoglutarate, mutant IDH generates an abnormal oncometabolite (a substance that when broken down, induces cancer) known as 2-hydroxyglutarate (2-HG). This 2-HG accumulation disrupts various cellular processes, including DNA methylation and histone modification, which causes the malignant transformation of cells. The altered epigenetic landscape promotes uncontrolled cell growth and inhibits normal cellular differentiation, which causes the development and progression of gliomas and other IDH-mutation related problems.
Vorasidenib, a potent IDH inhibitor, intervenes in this molecular cascade by selectively targeting the mutated IDH enzyme. By binding to the mutated enzyme's active site, Vorasidenib inhibits its aberrant catalytic activity. This, in turn, prevents the excessive production of 2-HG, mitigating the downstream effects on cellular processes. The disruption of 2-HG production serves as a key mechanism by which Vorasidenib hinders the progression of IDH-mutated cancers, offering a targeted therapeutic strategy that addresses the root cause of tumorigenesis (creation of tumors) at the molecular level.
So, what makes Vorasidenib unique? Aren’t there several medicines that can be used to block the same proteins? Like seriously, what’s the point of another drug that does the same thing as others?
Vorasidenib is a drug that can penetrate the blood-brain barrier, allowing for increased brain penetrance and thus increased efficiency in inhibiting the action of the IDH-1 and IDH-2 proteins, specifically the creation of the 2-HG molecule (see figure above). In essence, it’s way more efficient than these alternatives. But what does the data say? Rigorous studies have been conducted to assess the safety, efficacy, and tolerability of Vorasidenib, shedding light on its potential not only as a targeted therapy for IDH-mutated gliomas but for acute myeloid leukemia (AML) as well.
In pivotal phase III trials for gliomas, Vorasidenib has demonstrated a significant extension in progression-free survival, providing hope for patients grappling with these aggressive brain tumors. Human trials have also shown its role in AML treatment, and have had favorable outcomes in terms of survival and disease response rates. Published data from these trials highlight the drug's ability to effectively inhibit the mutated IDH enzyme, resulting in a substantial reduction in oncometabolite levels. Moreover, the trials underscore vorasidenib's manageable safety profile, with side effects such as nausea and fatigue being generally well-tolerated.
As these findings continue to be disseminated through peer-reviewed journals and scientific conferences, the accumulating evidence solidifies vorasidenib's standing as a promising therapeutic option. Through its targeted approach, the drug hones in on the aberrant IDH enzyme, and has showcased remarkable efficacy in slowing tumor growth. As modern medicine increases the efficiency of treatment methods for gliomas and AML, Vorasidenib's promising outcomes point toward more personalized and effective therapies. Encouragingly, the drug is on the brink of FDA approval, heralding a new era in precision medicine. With vorasidenib's impending regulatory recognition, there is newfound optimism for improved outcomes and enhanced quality of life for those facing these challenging diagnoses. Thank you all for reading, and as always, stay curious!
See y'all next time,
Parth and Chinmay